The atomic nucleus
03.00
Diposting oleh zakky amarullah
The nucleus of an atom contains nucleons: protons and neutrons
Nuclei are typically femtometres in diameter - (a femtometre (fm) is 10-15m). To view a scaled diagram of an atom on your computer screen with a nucleus of 1 cm diameter in the centre you would need a screen of 1km diameter to show the full graphic! The nucleus is tiny compared to a whole atom. It occupies one part in 100,000,000,000,000 of the atomic volume
The number of protons (Z) determines the chemical properties of the atom - which element it belongs to - it is called the proton number or atomic number.
The number of neutrons (N) affects its physical properties - which isotope of the element it belongs to.
The mass number (A) or nucleon number is the number of protons plus the number of neutrons
Various types of scattering experiments suggest that nuclei are roughly spherical and appear to have essentially the same average density.
This is studied at A level and is deduced from the following equation - where r is the radius of the nucleus of mass number A.:
![]()
This equation can be deduced from the following:
Mass of the nucleus will depend on A - the number of nucleons in the nucleus - total mass = Am
If the nucleus is spherical we have a volume ![]()
Therefore as m and ![]() are constant and density is mass / volume - density it is proprtional to A/r3
are constant and density is mass / volume - density it is proprtional to A/r3
and rearranging this we get that r is proportional to the density divided by the cube root of A
If we call the constant of proportionality ro we get the above equation.
If r is plotted against A1/3 a straight line is obtained - the gradient of it is the constant ro - but the relationship is best for large values of A so if you use small values this does not work too well!
---------------------------------------------------------------------------
To show that nuclear density is independent of A (or that all nuclei have the same density - or uniform density)
Let the volume of a nucleus be V
the mass of the nucleus be M
and the mass of a nucleon be m
V = 4/3![]() r3
r3
![]() = M/V
= M/V
So, M = 4/3![]() r3
r3![]()
Let's replace the r3 from the equation
![]()
r3 = ro3A
M = 4/3![]() ro3A
ro3A ![]()
but M = Am
So, Am = 4/3![]() ro3A
ro3A ![]()
Therefore,
Rearranging we get, ![]() = 3m/(4
= 3m/(4![]() ro3 )
ro3 )
This means thatthe density does not depednd on A or r - it is related to constant values - it is therefore a constant value.
______________________________________
The most definitive information about nuclear sizes comes from electron scattering experiments.
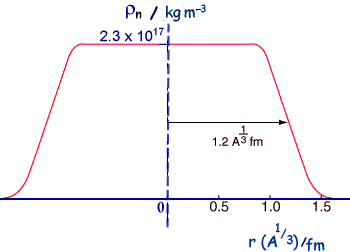 If we compare calculated and experimental radii for nuclei we find that that there is an area around the edge of the nucleus where the density of nuclear matter decreases toward zero - but that the central part is roughly of constant density. So the nucleus cannot be thought of as just a hard sphere.
If we compare calculated and experimental radii for nuclei we find that that there is an area around the edge of the nucleus where the density of nuclear matter decreases toward zero - but that the central part is roughly of constant density. So the nucleus cannot be thought of as just a hard sphere.
The fact that the nuclear density is independent of the details of neutron number or proton number suggests that the force between the particles is essentially the same whether they are protons or neutrons. This agrees with other evidence that the strong force is the same between any pair of nucleons.
Posting Komentar